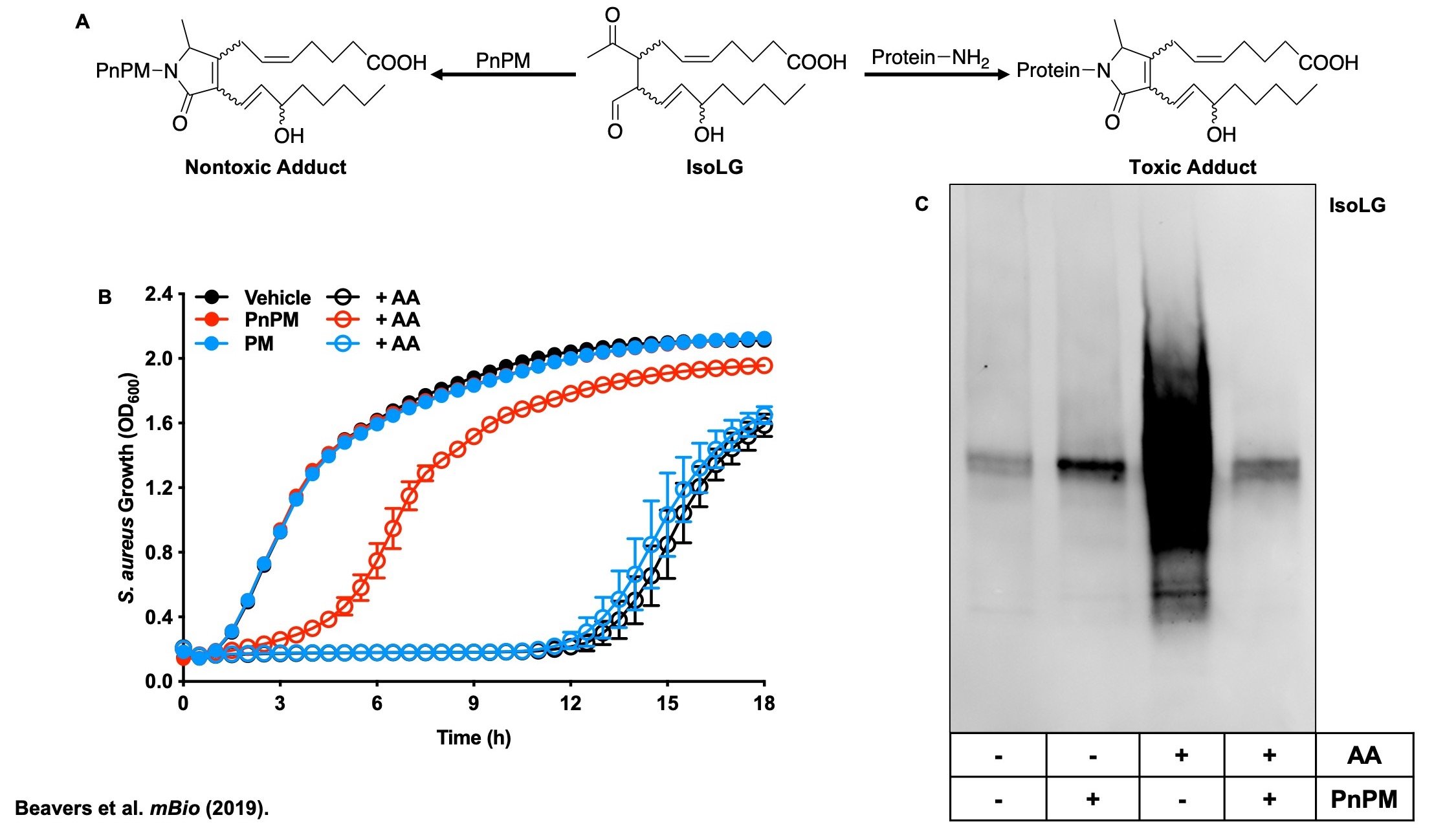
(A) Arachidonic acid (AA) kills S. aureus through a lipid peroxidation mechanism. One specific class of lipid electrophiles generated during lipid peroxidation are γ-ketoaldehydes such as isolevuglandins (IsoLG). These dicarbonyl lipid electrophiles are extremely reactive with primary amines including the ε-amine of lysine. Due to the pathogology associated with γ-ketoaldehydes in mammals, many tools were developed to facilitate their study. One set of tools are dicarbonyl scavengers derived from the pyridoxamine scaffold, which are 1000X more reactive with IsoLG than the ε-amine of lysine. (B) We used two different dicarbonyl scavengers to assess if IsoLG are being formed in S. aureus, and to determine where in the cell they are formed. Pyridoxamine (PM) and 5’-O-pentyl-pyridoxamine (PnPM) are equally reactive with IsoLG, but PM is hydrophilic and thus membrane impermeable, while PnPM readily crosses the membrane barrier due to its hydrophobicity. PnPM protects S. aureus from killing by AA, while PM does not, indicating that IsoLGs are bactericidal and that that autoxidation of AA occurs inside of the S. aureus cell. (C) An antibody that binds to IsoLG protein post-translational modifications (PTM) shows that IsoLG PTMs are not present in S. aureus before treatment with AA. IsoLG PTMs become abundant across a large swath of the S. aureus proteome following AA treatment. Co-treatment of S. aureus with AA and PnPM eliminates the IsoLG PTMs, correlating with the protection from the bactericidal activity of AA by PnPM seen in panel B. Together, this figure demonstrates that AA undergoes autoxidation in S. aureus, and that the lipid electrophiles generated during autoxidation are responsible for the bactericidal activity of AA against S. aureus.