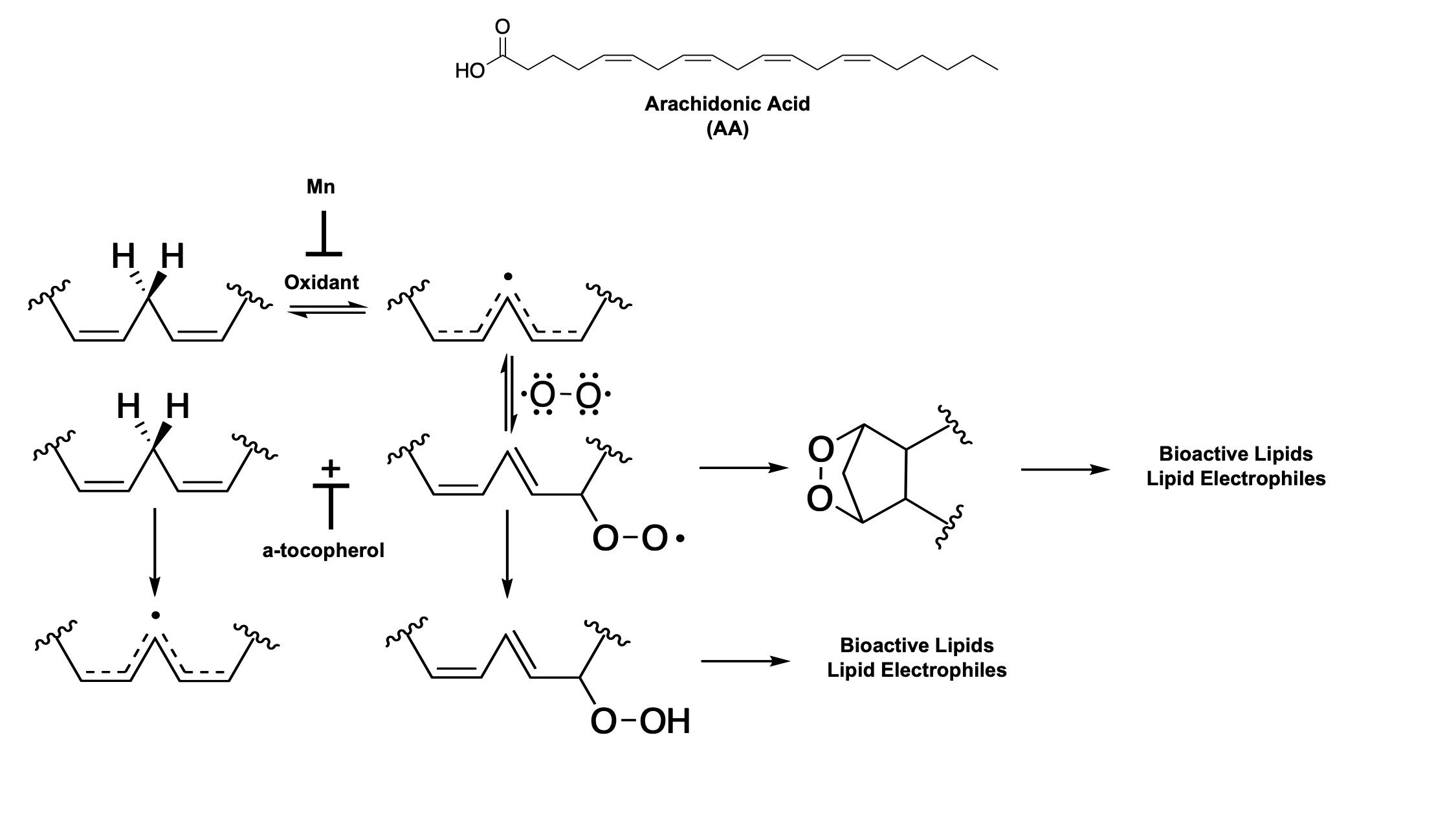
Arachidonic acid (AA) is an abundant, host polyunsaturated fatty acid (PUFA). It is toxic against S. aureus through a lipid peroxidation mechanism where AA is oxidized to various bactericidal lipid electrophiles. All PUFAs are susceptible to oxidation at the bis-allylic carbon (R1-CH=CH-CH2-CH=CH-R2). The carbon radical that results from hydrogen abstraction by an oxidant is stabilized through delocalization across five carbon atoms. Molecular oxygen, which exists as a diradical reacts with the carbon radical to form a lipid hydroperoxyl radical. The lipid hydroperoxyl radical can cyclize forming an endoperoxide and terminating the radical chain reaction. Further reactions involving the endoperoxide will form bioactive lipids such as isoprostanes and lipid electrophiles such as γ-ketoaldehydes. The lipid hydroperoxyl radical can also oxdize an adjacent PUFA, propagating the radical chain reaction and forming a lipid hydroperoxide. Furhter reactions involving the lipid hydroperoxide will form bioactive lipids such as hydroxyeicosatetraenoic acids (HETEs) and lipid electrophiles such as aldehydes and α,β-unsaturated carbonyls. Antioxidants can halt lipid peroxidation at multiple points. Manganese, which is used by S. aureus as an antioxidant, reduces the initiating oxidant, preventing lipid peroxidation. The strongest naturally occuring antioxidant, α-tocopherol, can reduce the lipid hydropoeroxyl radical forming a lipid hydroperoxide and preventing propagation of the radical chain reaction of lipid peroxidation.