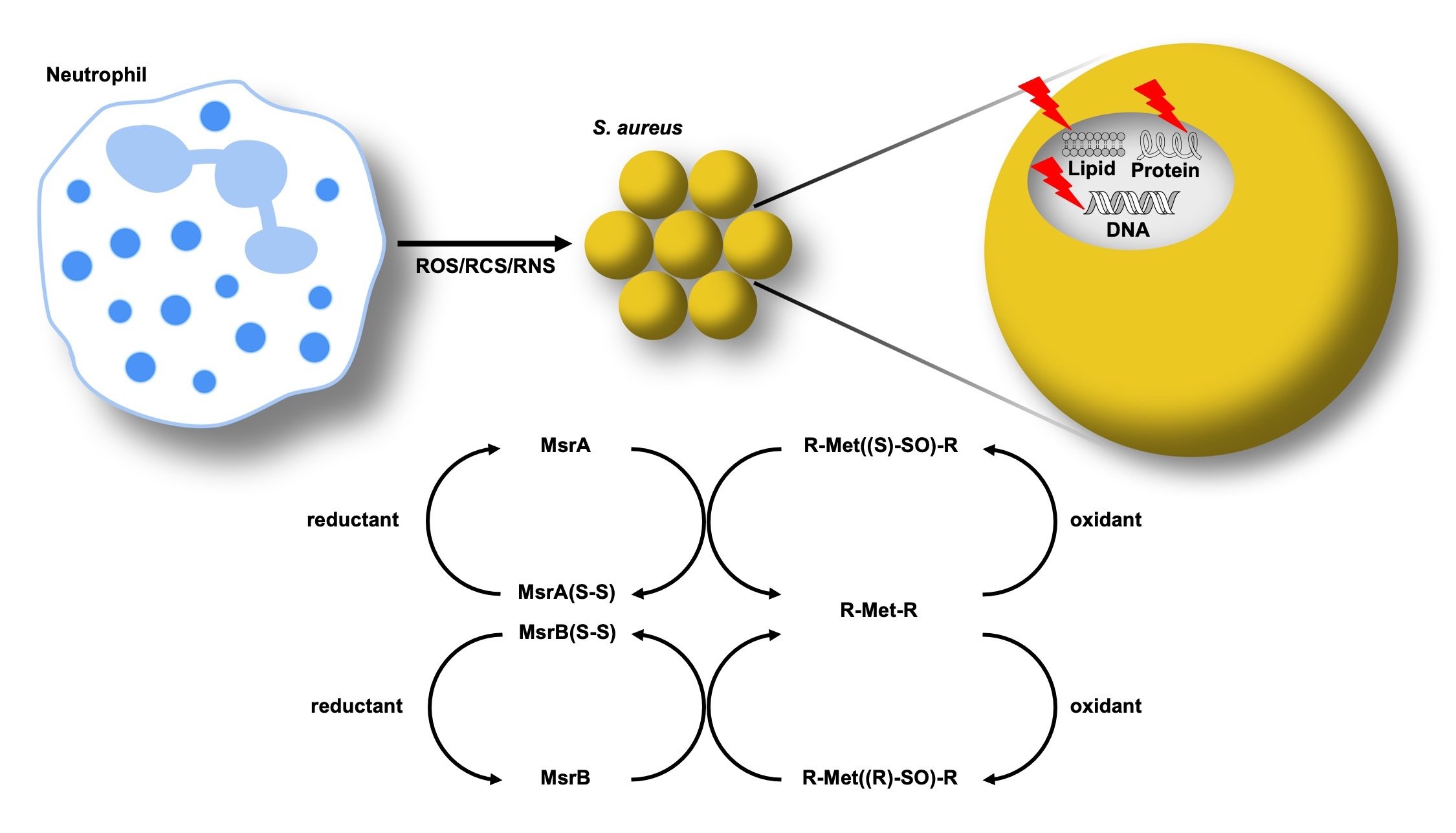
S. aureus is adept at avoiding and repairing oxidative damage to its cellular macromolecules caused by the host. Repair of oxidative damage to proteins provides a competitive advantage to the organism because if repair were not possible, damaged proteins would need to be resynthesized de novo following each deleterious oxidation event. This is a time and energy intensive process, especially when S. aureus is attempting to colonize and survive many other host antimicrobial stressors. The sulfur containing amino acids, cysteine and methionine, are particularly susceptible to oxidation. The thioredoxin and thioredoxin reductase system repairs oxidized cysteines, while the methionine sulfoxide reductases (Msr) revert methionine sulfoxide to methionine.
S. aureus is unique among bacterial pathogens in that it has four Msr enzymes (MsrA1, MsrA2, MsrA3, and MsrB). Methionine sulfoxide is a chiral molecule, and the A reductases have activity toward the (S)-epimer, while the B reductase has activity toward the (R)-epimer. Why S. aureus has three A reductases and one B reductase is unknown, but the enzymes are not fully redundant. Current projects in the lab seek to define the differences between the enzymes and discover their unique roles in S. aureus pathogenesis.