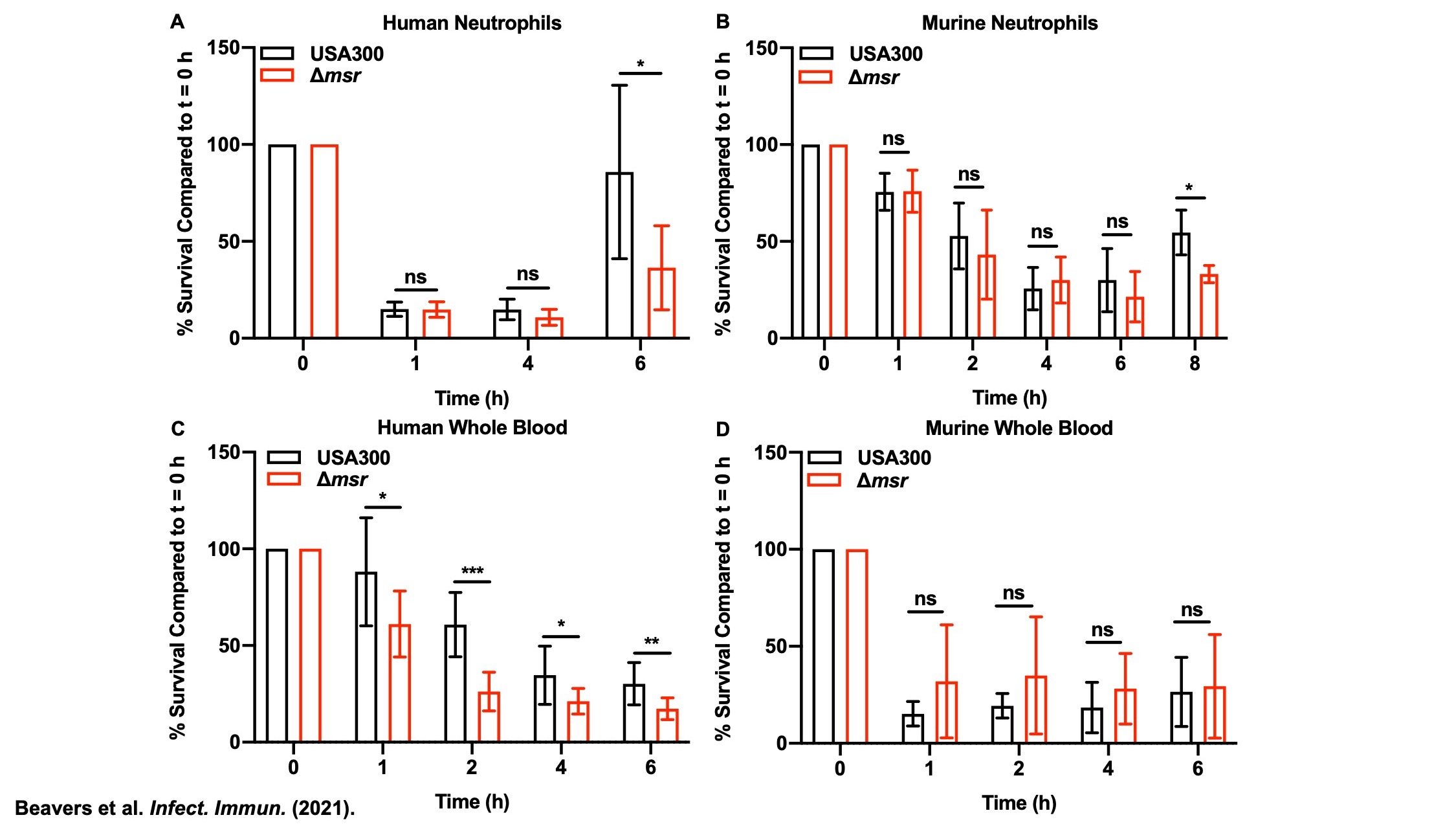
Methionine sulfoxide reductases (Msr) protect S. aureus against neutrophil killing and human whole blood killing, but not against murine whole blood killing. (A) USA300 wildtype and Δmsr were incubated with polymorphonuclear leukocytes (PMN) (MOI = 1) isolated from human blood. Killing by PMNs was assessed by plating for colony forming units (CFU) following the co-incubation. (B) USA300 wildtype and Δmsr were incubated with PMNs (MOI = 1) isolated from murine bone marrow. Killing by PMNs was assessed by plating for CFU following the co-incubation. USA300 wildtype recovers from co-incubation with PMNs better than Δmsr because Δmsr cannot repair oxidized methionine residues and must resynthesize oxidized proteins de novo before growth can resume. Both human and murine PMNs exhibit similar killing phenotypes when co-incubated with S. aureus, indicating no species differences in PMN function against S. aureus. (
C) USA300 wildtype and Δmsr were incubated with human whole blood and killing was assessed by plating for CFU. (D) USA300 wildtype and Δmsr were incubated with murine whole blood and killing was assessed by plating for CFU. Human whole blood kills Δmsr better than USA300 wildtype at all of the time points tested, but there is no difference between the strains in murine whole blood. Humans have 10-fold greater circulating neutrophils than mice, and this demonstrates that potentially the deficiency in circulating neutrophils in murine blood renders the Msr enzymes less important for pathogenesis in a murine host, consistent with our other findings.